Interfacial interactions and structures of protic ionic liquids on a graphite surface: A first-principles study and comparison with aprotic ionic liquids†
Abstract
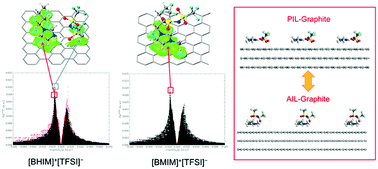
Protic ionic liquids (PILs) have currently been indicated as promising alternative electrolytes in electrical storage devices, such as lithium-ion batteries and supercapacitors. However, compared with the well-studied aprotic ionic liquids (AILs), the knowledge of the interface between PILs and electrode material surfaces is very rare to date. In this work, the adsorption behaviors of three groups of PILs, i.e. pyrrolidinium-based, imidazolium-based, and ammonium-based, on graphite was systematically investigated using first-principles calculations. The corresponding AILs were also taken into account for comparison. The adsorption mechanism of these ILs on the surface is controlled by the interplay of strong electrostatic interactions between adsorbed ions, weak vdW forces between ILs and substrate, and many aromatic interactions including π–π stacking and C–H/N–H⋯π contacts. PILs do show quite different preferential interfacial interactions and structures on the graphite surface with respect to AILs, arising mainly from the anion–substrate interactions. Particularly, proton transfer takes place in the PILs consisting of the imidazolium/ammonium cation and the nitrate anion in the gas phase, but it tends to be attenuated or even disappears on graphite caused by interfacial interactions.
- This article is part of the themed collection: 2021 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...