Ab initio rate coefficients for reactions of 2,5-dimethylhexyl isomers with O2: temperature- and pressure-dependent branching ratios†
Abstract
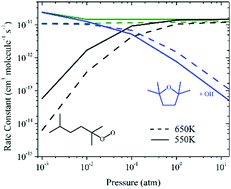
Chemical kinetics of O2-addition to alkyl radicals (R), termed first O2-addition in the oxidation mechanism of alkanes, are of central importance to next-generation combustion strategies designed for operations in the low- to intermediate-temperature region (<1000 K). In the present work, stationary points on potential energy surfaces (PES), temperature- and pressure-dependent rate coefficients, and branching fractions of product formation from R + O2 reactions initiated by the addition of molecular oxygen (3O2) to the three alkyl radicals of a branched alkane, 2,5-dimethylhexane, are reported. The stationary points were determined utilizing ab initio/DFT methods and the reaction energies were computed using the composite CBS-QB3 method. Rice–Ramsperger–Kassel–Marcus (RRKM)/master equation (ME) calculations were employed to compute rate coefficients, from which branching fractions were determined over the pressure range of 10−3–20 atm and the temperature range of 400–900 K on three different surfaces. The quantum chemistry results reveal several distinct features. For the addition of O2 to the tertiary alkyl radical 2,5-dimethylhex-2-yl, the most energetically favorable channel leads to the formation of 2,2,5,5,-tetramethyl-tetrahydrofuran, a cyclic ether intermediate formed coincident with OH in a chain-propagating step from the decomposition of tertiary–tertiary hydroperoxyalkyl (QOOH). On the R + O2 surface of the secondary radical, 2,5-dimethylhex-3-yl, the pathways for the formation of methyl-propanal + iso-butene + OH via concerted C–C and O–O bond scission of tertiary QOOH and that of cyclic ether + OH are the most energetically favorable pathways. The R + O2 surface for the reaction of the primary radical, 2,5-dimethylhex-1-yl, reveals two competitive chain-propagation channels, leading to 2-iso-propyl-4-methyl-tetrahydrofuran + OH and 2,2,5-trimethyltetrahydropyran + OH. Below 100 Torr, the formation of the aforementioned species dominates the respective total R + O2 rate coefficient, while at pressures above 1 atm collisionally stabilized alkylperoxy (ROO) dominates at the temperatures considered here. The results of this study are in very good agreement with the experimentally measured intermediates and products of the 2,5-dimethylhexyl radical + O2 reaction.


 Please wait while we load your content...
Please wait while we load your content...