Insights into the hydrogen bond network topology of phosphoric acid and water systems†
Abstract
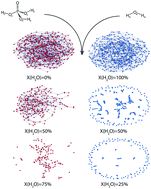
Phosphoric acid and its mixtures with water are some of the best proton conducting materials known to science. Although the proton conductivity in pure phosphoric acid decreases upon external doping with excess H+ or OH−, the addition of water improves it substantially. A number of experimental and theoretical studies indicate that these systems form a very special case of hydrogen bond networks which not only facilitate fast proton transport but also show a number of other interesting properties such as glass forming ability. In this work, we present the molecular dynamics simulation results of the H3PO4–H2O system over the entire concentration range. The hydrogen bond networks were analyzed in terms of conventional microscopic as well as topological properties based on graph and network theory. The results show that the hydrogen bond network of H3PO4 is fundamentally different from that of H2O. On average, each phosphoric acid molecule tends to form more and stronger hydrogen bonds than water which leads to a much more connected and clustered network showing small-world properties which are absent in pure water. Moreover, these hydrogen bond network properties persist in the H3PO4–H2O mixtures as well, even at relatively high water contents. Finally, many of the physical properties such as molecular diffusion coefficients seem to be also intimately related to the network topological properties and follow similar trends with respect to system content. These results strongly indicate that many important properties such as proton transport in phosphoric acid and its aqueous systems are fundamentally related to their hydrogen bond network topology and might hold the key for their ultimate molecular understanding.


 Please wait while we load your content...
Please wait while we load your content...