A theoretical study on the role of ammonium ions in the double-layered V2O5 electrode
Abstract
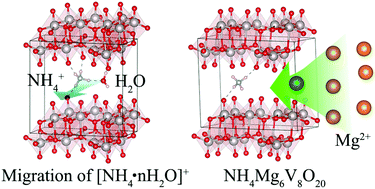
Double-layered V2O5 and its analogues have received increasing attention as a proper cathode for Mg2+, Na+, Li+ ion batteries, even for ammonium ion batteries. Our theoretical research focuses on the effects of NH4+ ions on the structural stability and the ion diffusion properties of double-layered V2O5. The elastic constant calculations indicate the NH4+ and water contents have a dramatic influence on the stability of the electrode. When the ratio of H2O and ammonia ions decreases to (NH4)0.125V2O5·0.125H2O, double-layered bronze will transform into other phases. The predicted specific capacity for the redox process from (NH4)0.5V2O5·0.5H2O to (NH4)0.125V2O5·0.125H2O is 54.6 mA h g−1, which agrees with the experimental value of 55.6 mA h g−1. From the diffusion barrier calculations, it is found that the H2O molecules can shield the polarization of NH4+ and lower the diffusion barrier of NH4+ ions. Furthermore, the migrations of common charge carriers in NH4+ pre-intercalated V2O5 have also been studied, which implies that Li+, Zn2+, Na+, Mg2+ ions may move easily in the electrode with energy barriers lower than 525 meV. Our findings match well with the reported experimental results. A special structure of Mg6NH4V8O20 with a much higher Mg ion concentration has been reported. Our findings show that the theoretical specific density of Mg batteries based on NH4+ pre-intercalated V2O5 can be improved to 431 mA h g−1, which is 2.5 times larger than the reported values. This work highlights the effects of the ratio of NH4+ and H2O on double-layered V2O5 and provides insights into designing vanadium oxide based fast-diffusion multivalent ion conductors, which are suitable for battery applications.


 Please wait while we load your content...
Please wait while we load your content...