Electric field-driven conformational changes in the elastin protein
Abstract
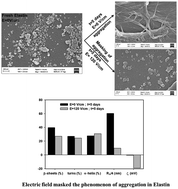
The formation of aggregates and amyloids, a hallmark of many protein misfolding diseases, depends on many intrinsic and extrinsic factors. Many approaches (in vitro, in vivo, and in silico) have been attempted to inhibit the aggregation process so that the progression of these diseases can be controlled. We investigate the effect of a static electric field (EF; 120 V cm−1 and 200 V cm−1) on the conformational change of elastin protein using light scattering, spectroscopy, and microscopy techniques. Laser light scattering and photoluminescence spectroscopy show the formation of fibrils of unexposed elastin with aging, whereas disruption of fibril formation with EF exposed elastin. The size of EF exposed elastin first increases and exhibits an apex, and subsequently decreases with an increasing time of exposure. We observed that a decrease in the size of EF exposed elastin depends on the strength of the EF, faster decrement at higher EF. FTIR data show that EF modifies elastin protein's secondary structures; it facilitates the interconversion of β-sheets and turns into α-helix structures. The SEM images of unexposed and EF exposed elastin confirms the observation through light scattering and PL techniques. The effect of an EF on protein conformation and amyloids is promising to treat Parkinson's disease, a protein misfolding disease.


 Please wait while we load your content...
Please wait while we load your content...