Formation of silaimines from a sterically demanding iminophosphonamido chlorosilylene via intramolecular N–P bond cleavage†
Abstract
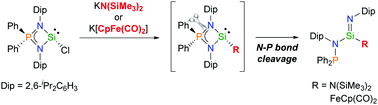
The sterically demanding iminophosphonamido chlorosilylene [Ph2P(DipN)2]SiCl (Dip = 2,6-diisopropylphenyl) was synthesized and fully characterized using NMR spectroscopy and X-ray crystallography. Substitution reactions of [Ph2P(DipN)2]SiCl with N- and Fe-nucleophiles led to the unexpected formation of the corresponding silaimine derivatives. This process involves the ring-opening rearrangement of three-coordinated silylene intermediates that proceeds via intramolecular N–P bond cleavage.


 Please wait while we load your content...
Please wait while we load your content...