Cubane-forming cyclic dienes that exhibit orthogonal reactivities in the solid state†
Abstract
Photoirradiation of a binary cocrystal composed of two different cyclic dienes generates a highly-symmetric cubane-like tetraacid cage regioselectively and in quantitative yield. The cage forms by a double [2+2] photodimerization of one of the diene cocrystal components. The second diene while photostable in the cocrystal reacts in a double [2+2] photodimerization as a pure form quantitatively to form a tetramethyl cubane-like cage. The stereochemistry of the cage is structurally authenticated.
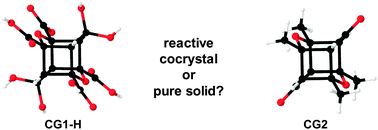


 Please wait while we load your content...
Please wait while we load your content...