Synthesis of a novel cyclopropyl phosphonate nucleotide as a phosphate mimic†
Abstract
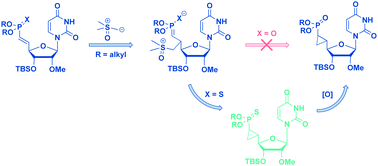
The inherent in vivo instability of oligonucleotides presents one of many challenges in the development of RNAi-based therapeutics. Chemical modification to the 5′-terminus serves as an existing paradigm which can make phosphorylated antisense strands less prone to degradation by endogenous enzymes. It has been recently shown that installation of 5′-cyclopropyl phosphonate on the terminus of an oligonucleotide results in greater knockdown of a targeted protein when compared to its unmodified phosphate derivative. In this paper we report the synthesis of a 5′-modified uridine.


 Please wait while we load your content...
Please wait while we load your content...