Iron-catalyzed stereoselective haloamidation of amide-tethered alkynes†
Abstract
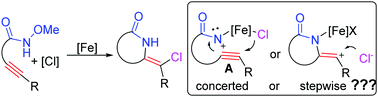
In this work, by using N-methoxybenzamides as efficient acyl nitrene precursors, an iron-catalyzed formal cis-haloamidation of alkyne is reported. Without assistance of additives, the reaction worked well in the presence of 50 mol% FeCl3 or FeBr3, leading to a series of chloro/bromo-containing isoindolin-5-ones with high efficiency and wide reaction scope. In the reaction, the iron-facilitated haloamidation proceeds through a halo anion-participating concerted [3+2] cyclization to release the final products. The key intermediate ferric acyl nitrene A is generated in situ from a formal removal of MeOH.


 Please wait while we load your content...
Please wait while we load your content...