Overcoming the phase separation within high-entropy metal carbide by poly(ionic liquid)s†
Abstract
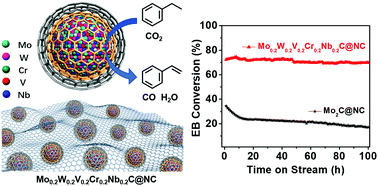
High-entropy crystalline materials are attracting more attention. In principle, high-entropy metal carbides (HMCs) that contain five or more metal ions, possess more negative free energy value during catalysis. But its preparation is challenging because of the immiscibility of multi metal cations in a single carbide solid solution. Here, a rational strategy for preparing HMC is proposed via a coordination-assisted crystallization process in the presence of Br-based poly(ionic liquids). Through this method, Mo0.2W0.2V0.2Cr0.2Nb0.2C nanoparticles, with a single cubic phase structure, incorporated on porous carbon, are obtained (HMC@NC). By combination of well dispersed small particle size (∼4 nm), high surface area (∼270 m2 g−1), and high-entropy phase, HMC@NC can function as a promising catalyst for the dehydrogenation of ethylbenzene. Unexpected activity (EB conv.: 73%) and thermal stability (>100 h on steam) at 450 °C are observed. Such a facile synthetic strategy may inspire the fabrication of other types of HMCs for more specific tasks.


 Please wait while we load your content...
Please wait while we load your content...