Transition metal-mediated B(4)–H hydroxylation/halogenation of o-carboranes bearing a 2-pyridylsulfenyl ligand†
Abstract
The introduction of the 2-pyridylsulfenyl directing group to o-carboranes allowed either B(3)–Ir or B(4)–Ir bond formation using a steric effect strategy. Moreover, the reactivity of the B(4)–Rh o-carborane complexes with small molecules was probed by reactions with N-bromosuccinimide, N-iodosuccinimide and O2. Rhodium-mediated B(4)-hydroxylation and B(4)-halogenation which are seldom reported have been achieved under practical and mild conditions.
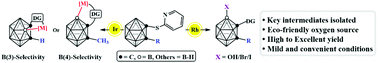


 Please wait while we load your content...
Please wait while we load your content...