Abstract
Fully aromatic, luminescent, and highly robust BNB-doped phenalenyls have been prepared, which are isoelectronic to the phenalenyl cation. B-Fluoromesityl-substitution leads to fluorescence in an extremely narrow range and significant increase in the reduction potential. Detailed theoretical investigations revealed an intramolecular aromaticity switch upon one-electron reduction.
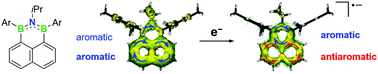


 Please wait while we load your content...
Please wait while we load your content...