Oxidation triggers guest dissociation during reorganization of an Fe II4L6 twisted parallelogram†
Abstract
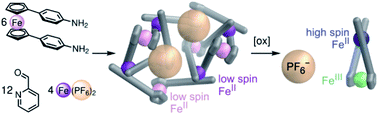
A three-dimensional FeII4L6 parallelogram was prepared from ferrocene-containing ditopic ligands. The steric preference of the bulky ferrocene cores towards meridional vertex coordination brought about this new structure type, in which the ferrocene units adopt three distinct conformations. The structure possesses two distinct, bowl-like cavities that host anionic guests. Oxidation of the ferrocene FeII to ferrocenium FeIII causes rotation of the ferrocene hinges, converting the structure to an FeII1L1+ species with release of anionic guests, even though the average charge per iron increases in a way that would ordinarily increase guest binding strength. The degrees of freedom exhibited by these new structures – derived from the different configurations of the three ligands surrounding a meridional FeII center and the rotation of ferrocene cores – thus underpin their ability to reconfigure and eject guests upon oxidation.


 Please wait while we load your content...
Please wait while we load your content...