Nitric oxide monooxygenation (NOM) reaction of cobalt-nitrosyl {Co(NO)}8 to CoII-nitrito {CoII(NO2−)}: base induced hydrogen gas (H2) evolution†
Abstract
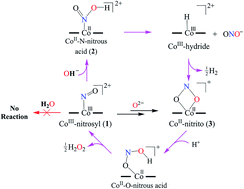
Here, we report the nitric oxide monooxygenation (NOM) reactions of a CoIII-nitrosyl complex (1, {Co-NO}8) in the presence of mono-oxygen reactive species, i.e., a base (OH−, tetrabutylammonium hydroxide (TBAOH) or NaOH/15-crown-5), an oxide (O2− or Na2O/15-crown-5) and water (H2O). The reaction of 1 with OH− produces a CoII-nitrito complex {3, (CoII-NO2−)} and hydrogen gas (H2), via the formation of a putative N-bound Co-nitrous acid intermediate (2, {Co-NOOH}+). The homolytic cleavage of the O–H bond of proposed [Co-NOOH]+ releases H2via a presumed CoIII-H intermediate. In another reaction, 1 generates CoII-NO2− when reacted with O2−via an expected CoI-nitro (4) intermediate. However, complex 1 is found to be unreactive towards H2O. Mechanistic investigations using 15N-labeled-15NO and 2H-labeled-NaO2H (NaOD) evidently revealed that the N-atom in CoII-NO2− and the H-atom in H2 gas are derived from the nitrosyl ligand and OH− moiety, respectively.


 Please wait while we load your content...
Please wait while we load your content...