Dynamic reaction-induced phase separation in tunable, adaptive covalent networks†
Abstract
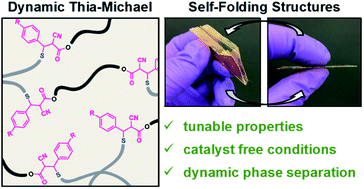
A series of catalyst-free, room temperature dynamic bonds derived from a reversible thia-Michael reaction are utilized to access mechanically robust dynamic covalent network films. The equilibrium of the thiol addition to benzalcyanoacetate-based Michael-acceptors can be directly tuned by controlling the electron-donating/withdrawing nature of the Michael-acceptor. By modulating the composition of different Michael-acceptors in a dynamic covalent network, a wide range of mechanical properties and thermal responses can be realized. Additionally, the reported systems phase-separate in a process, coined dynamic reaction-induced phase separation (DRIPS), that yields reconfigurable phase morphologies and reprogrammable shape-memory behaviour as highlighted by the heat-induced folding of a predetermined structure.


 Please wait while we load your content...
Please wait while we load your content...