meta-Selective olefination of fluoroarenes with alkynes using CO2 as a traceless directing group†
Abstract
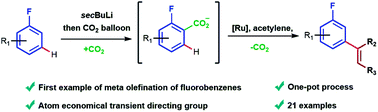
Over the last few decades C–H olefination has received significant interest, due to the importance and usefulness of aryl olefins both as synthetic targets and intermediates. While a wide range of ortho-olefination protocols have been developed, only a small number of meta-olefinations are currently available. Importantly, the most common approach to meta-olefination, using a large meta-directing template, is not suitable for substrates such as fluorobenzenes, which cannot be derivatised. We report that the meta-selective olefination of fluoroarenes can be achieved via the use of CO2 as a traceless directing group, which can be easily installed and removed in a one-pot process. Furthermore, this approach avoids the use of stoichiometric Ag(I)-salts, commonly used in C–H olefinations, and affords complete meta- over ortho/para-regioselectivity.


 Please wait while we load your content...
Please wait while we load your content...