Skeletal diversity in Pt- and Au-catalyzed annulations of allenedienes: dissecting unconventional mechanistic pathways†
Abstract
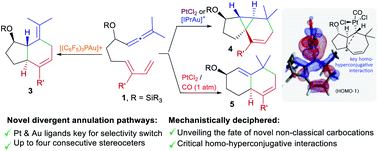
We describe the discovery of unprecedented annulation processes of 1,7-allenedienes, promoted by Pt or Au catalysts. These transformations revealed mechanistic pathways that had not been previously observed in reactions involving carbophilic catalysis. In particular, we have found that allenedienes bearing a silyl ether in the carbon tether connecting the diene and the allene divergently afford cyclopropane-embedded tricyclic derivatives, 6,6-fused bicarbocyclic products or 5,6-fused bicarbocyclic systems, depending on the type of Au or Pt catalyst used. We have carried out experimental and computational studies that shed light on the mechanistic reasons behind this rich and unusual skeletal divergence, and provide new lessons on the drastic influence of platinum ancillary ligands on the reaction outcome.


 Please wait while we load your content...
Please wait while we load your content...