FeS–biochar and Zn(0)–biochar for remediation of redox-reactive contaminants†
Abstract
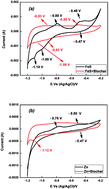
To enhance the removal of redox-reactive contaminants, biochars including FeS and Zn(0) were developed via pyrolysis. These biochars significantly promoted the removal of 2,4-dichlorophenol (DCP) by means of sorption and reduction. Compared to direct reduction with FeS and Zn(0), the formation of reduction intermediates and product was enhanced from 21% and 22% of initial DCP concentration to 41% and 52%, respectively. 2,4-Dinitrotoluene (DNT), chromate (CrO42−) and selenate (SeO42−) were also reductively transformed to reduction products (e.g., 2,4-diaminotoluene [DAT], Cr3+, and selenite [SeO32−]) after they sorbed onto the biochars including FeS and Zn(0). Mass recovery as DAT, Cr3+ and selenite was 4–20%, 1–3%, and 10–30% under the given conditions. Electrochemical and X-ray analyses confirmed the reduction capability of the biochars including FeS and Zn(0). Fe and S in the FeS–biochar did not effectively promote the reductive transformation of the contaminants. Contrastingly, the stronger reducer Zn(0) yielded faster reductive transformation of contaminants over the Zn(0)-containing biochar, while not releasing high concentrations of Zn2+ into the aqueous phase. Our results suggest that biochars including Zn(0) may be suitable as dual sorbents/reductants to remediate redox-reactive contaminants in natural environments.


 Please wait while we load your content...
Please wait while we load your content...