Ni stabilized rock-salt structured CoO; Co1−xNixO: tuning of eg electrons to develop a novel OER catalyst†
Abstract
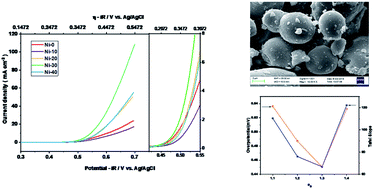
The oxygen evolution reaction (OER) is a key half-reaction in hydrogen–oxygen electrolysers that is very important for efficient electrochemical energy generation, storage and fuel production that offers a clean alternative to fissile fuel combustion based energy systems. Several transition metal containing perovskites were recently explored for the development of superior OER catalysts, and their activity was correlated with the applied potentials at a specific current density to eg electron density present in the materials. The rock salt structure is envisaged here as a model host structure similar to perovskite to tune the eg electrons to obtain superior electro-catalytic activity. Incorporation of Ni into CoO lattices helps to stabilize the rock salt structure and modulate the eg electrons to develop superior OER and ORR electrocatalysts. Nickel doped rock salt structured CoO, NixCo1−xO (0 ≤ x ≤ 0.5), were synthesized by employing a solid state metathesis synthesis route. The compounds were characterised by powder X-ray diffraction (XRD), TGA, FT-IR and X-ray Photoelectron Spectroscopy (XPS). Ni0.3Co0.7O with 1.3 eg electrons showed superior electrocatalytic activity for the oxygen evolution reaction. The overpotential for the Ni0.3Co0.7O sample was also found to be ∼0.450 V for 1 M and about ∼0.389 V at 5 M concentration of the KOH electrolyte.


 Please wait while we load your content...
Please wait while we load your content...