Cu/TEMPO catalyzed dehydrogenative 1,3-dipolar cycloaddition in the synthesis of spirooxindoles as potential antidiabetic agents†
Abstract
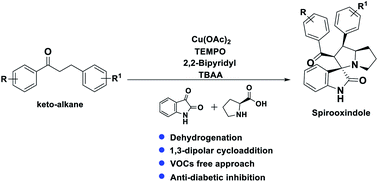
A series of spiro-[indoline-3,3′-pyrrolizin/pyrrolidin]-2-ones, 4, 5 and 6 were synthesized in a sequential manner from Cu–TEMPO catalyzed dehydrogenation of alkylated ketones, 1 followed by 1,3-dipolar cycloaddition of azomethine ylides via decarboxylative condensation of isatin, 2 and L-proline/sarcosine, 3 in high regioselectivities and yields. The detailed mechanistic studies were performed to identify the reaction intermediates, which revealed that the reaction proceeds via dehydrogenative cycloaddition. Additionally, the regio and stereochemistry of the synthesized derivatives were affirmed by 2D NMR spectroscopic studies. The synthesized derivatives were explored further with molecular docking, in vitro antioxidant, and anti-diabetic activities.


 Please wait while we load your content...
Please wait while we load your content...