Methane hydrate formation in an oil–water system in the presence of lauroylamide propylbetaine
Abstract
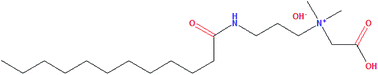
To enhance our understanding of the influence of quaternary ammonium salts on CH4 hydrate formation, the chosen anti-agglomerant lauroylamide propylbetaine (LPB) was tested in an oil–water system in this work and analyzed by Raman spectroscopy, PXRD, and SEM. The results showed that LPB promoted CH4 hydrate formation by reducing the induction time and increasing the CH4 consumption rate for hydrate growth. The promotion effect on the CH4 hydrate growth was the best when the LPB concentration reached 0.18 wt%. Raman and PXRD analyses of the hydrate samples showed that the ratio of the CH4 molecules in large and small cages was below 3 and the (222) plane of the CH4 hydrate formed from an LPB solution was obviously lower compared to that for a typical CH4 hydrate. It was suggested that the positions of the water molecules in the host water lattice changed. The LPB molecules were thought to modify the surface structure of the hydrate phase, where the methyl head groups of LPB were allowed to penetrate both the 51262 and 512 cages of the CH4 hydrate. The modifications on the hydrate surface were further revealed by SEM images. The porous surface of the formed solids turned into curved sheets when LPB was added. Therefore, the mechanical properties of the bulk solid phase were assumed to be weakened.


 Please wait while we load your content...
Please wait while we load your content...