Formation of cyclic structures in the cationic ring-opening polymerization of 1,3-dioxolane†
Abstract
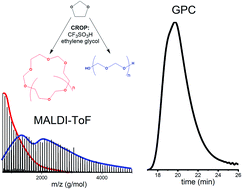
The cationic ring-opening polymerization of acetals is prone to cyclization of the polymer chains. This is also the case for the polymerization of 1,3-dioxolane. Literature states that this cyclization can be reduced by applying the Active Monomer mechanism, at least if no competition with the Active Chain End mechanism occurs. In this work, a detailed characterization of the different distributions resulting from the cationic ring-opening polymerization of 1,3-dioxolane via the Active Monomer mechanism is made by a combination of gel permeation chromatography, 1H NMR, and for the first time by matrix assisted laser desorption/ionization time of flight mass spectrometry. The influence of monomer addition speed, catalyst to initiator ratio and solvent were studied on both kinetics and composition of the product. Furthermore, it was found that increasing the conversion and monomer to initiator ratios leads to an increased amount of cyclic structures and to broader distributions, in correspondence with the Jacobson–Stockmayer theory. Furthermore, ion trapping experiments using 31P NMR provide insights into the actual reaction mechanism. Finally, purification of the products after the reactions led to a reduction of the cyclic fraction.


 Please wait while we load your content...
Please wait while we load your content...