Design and evaluation of novel thrombin-based GLP-1 analogs with peptidic albumin binding domain for the controlled release of GLP-1
Abstract
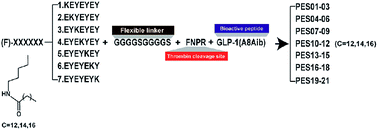
Currently, the curative effects of polypeptide drugs are often restricted due to the short in vivo duration of action. In this study, we fused a series of heptapeptide tags with different length fatty chains to the N-terminus of mutated glucagon-like peptide-1 (GLP-1) using an intermediate sequence comprising a flexible linker (GGGGS)2 and thrombin (TBN)-cleavable site (FNPR), to develop promising prolonged GLP-1 receptor (GLP-1R) agonists. As a result, twenty-one fusion peptides, termed PES01–PES21, were designed and prepared. Surface plasmon resonance (SPR) measurements and plasma stability tests showed that PES14 exert better albumin binding affinity and in vitro plasma stability compared with the other ones. Preclinical assay in db/db mice proved that PES14 exert the hypoglycemic efficacies in a dose-dependent model within the range of 10–90 nmol kg−1. Furtherly, an enhanced glucose-lowering effect and significantly prolonged hypoglycemic duration of PES14 were exhibited in multiple oral glucose tolerance tests (OGTTs) and hypoglycemic duration test, compared with Liraglutide and Semaglutide, respectively. Moreover, the in vivo t1/2 of intact PES14 and released GLP-1 were approximately 95.1 h and 110.5 h in rhesus monkeys after a single subcutaneous injection of 90 nmol kg−1, respectively. Furthermore, long-term treatment with PES14 in db/db mice for 8 weeks obtained beneficial efficacies on body weight gain, food intake, fat% and hemoglobin A1c (HbA1c) reduction compared with the control and superior to those of Semaglutide treatment. Meanwhile, chronic treatment of PES14 also exhibited proper insulin immunoreactivity and effectively enhanced the improvement on hepatocyte damage. All these results suggested that PES14 has the potential to be developed as a once-weekly anti-diabetic drug.


 Please wait while we load your content...
Please wait while we load your content...