Determination of acid dissociation constants of Alizarin Red S, Methyl Orange, Bromothymol Blue and Bromophenol Blue using a digital camera†
Abstract
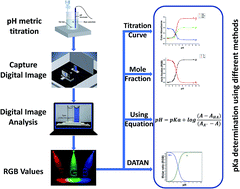
Acid dissociation constants (pKa) are important parameters for the characterization of organic and inorganic compounds. They play a crucial role in different physical, chemical, and biological studies. Herein, we introduce a new approach for the determination of acid dissociation constant based on digital image analysis using a low-cost, precise, accurate, sensitive, and portable home-made, camera-based platform. Digital images of Alizarin Red S, Bromophenol Blue, Bromothymol Blue, and Methyl Orange solutions were captured at various pH values. The captured images were analysed to obtain the RGB (Red, Green, and Blue) colour intensities that are used to calculate the RGB colour absorbances. The pKa values were calculated from the RGB colour absorbance–pH relationship using graphical and mathematical methods, and with the aid of DATAN software. For the four studied dyes, the results obtained from digital image analysis were in excellent agreement with the data of sophisticated spectrophotometers and the previously reported literature data.


 Please wait while we load your content...
Please wait while we load your content...