Thermochemistry of cation disordered Li ion battery cathode materials,
 (M′ = Nb and Ta, M′′ = Mn and Fe)†
(M′ = Nb and Ta, M′′ = Mn and Fe)†
Abstract
High temperature oxide melt solution calorimetry studies on 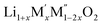 (M′ = Nb5+, M′′ = Mn3+ and Fe3+ and x = 0.20, 0.30 and 0.40) oxides and a new family of Ta containing Li excess disordered cathode materials,
(M′ = Nb5+, M′′ = Mn3+ and Fe3+ and x = 0.20, 0.30 and 0.40) oxides and a new family of Ta containing Li excess disordered cathode materials, 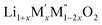 (M′ = Ta5+, M′′ = Fe3+ and x = 0.20, 0.30 and 0.40), synthesized by a rapid quenching method, are reported in this study. The enthalpies of formation determined from high temperature calorimetry studies reveal that the stability of compounds increases with the increasing Li content per formula unit. The reaction between more basic Li2O and acidic transition metal oxides results in the more negative enthalpies of formation for these compounds. The work reveals that the formation enthalpy term
(M′ = Ta5+, M′′ = Fe3+ and x = 0.20, 0.30 and 0.40), synthesized by a rapid quenching method, are reported in this study. The enthalpies of formation determined from high temperature calorimetry studies reveal that the stability of compounds increases with the increasing Li content per formula unit. The reaction between more basic Li2O and acidic transition metal oxides results in the more negative enthalpies of formation for these compounds. The work reveals that the formation enthalpy term 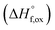 plays a more important role in the stabilization of such disordered Li ion materials at room temperature whereas configurational entropy along with lattice entropy (vibrational and magnetic) contributes to the stabilization at high temperature from which the samples are quenched.
plays a more important role in the stabilization of such disordered Li ion materials at room temperature whereas configurational entropy along with lattice entropy (vibrational and magnetic) contributes to the stabilization at high temperature from which the samples are quenched.



 Please wait while we load your content...
Please wait while we load your content...
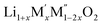 (M′ = Nb and Ta, M′′ = Mn and Fe)
(M′ = Nb and Ta, M′′ = Mn and Fe)