Direct synthesis of annulated indoles through palladium-catalyzed double alkylations†
Abstract
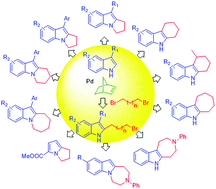
A facile, one-step synthesis of annulated indoles from (N–H) indoles and dibromoalkanes was developed through a palladium-catalyzed double alkylation process. The reaction proceeds through a palladium-catalyzed norbornene-mediated C2-alkylation of indoles and subsequent regioselective cyclization. The reaction is compatible with various indole substituents, as well as a range of simple and functionalized dibromoalkyl reagents. The detailed reaction mechanism was investigated by key intermediate experiments, NMR spectroscopic analyses, and DFT calculations. The results of a preliminary mechanistic study show a catalytic cycle consisting of initial C2-alkylation of indoles that is followed by N-alkylation or palladium promoted intramolecular C3-nucleophilic substitution to yield, respectively, [a]- or [b]-annulated indoles.


 Please wait while we load your content...
Please wait while we load your content...