One-step synthesis of MnOx/PPy nanocomposite as a high-performance cathode for a rechargeable zinc-ion battery and insight into its energy storage mechanism†
Abstract
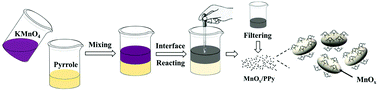
Rechargeable aqueous zinc-ion batteries (ZIBs) have attracted significant attention in the energy storage field. Manganese-based materials are the most promising cathode materials for ZIBs but they suffer from low electronic conductivity. Herein, a high-performance cathode for ZIBs based on nanocomposites consisting of mixed-valence manganese dioxide (Mn III and IV) and polypyrrole (MnOx/PPy) is prepared through an efficient one-step organic/inorganic interface redox reaction. The role of polypyrrole (PPy) in the MnOx/PPy cathode is elaborated. It not only provides an effective conductive network for MnOx but also contributes to the capacity of the composite. By optimizing the amount of PPy, the MnOx/PPy composite with 12 wt% PPy exhibits the highest capacity. As a result, the corresponding Zn-MnOx/PPy battery delivers a high capacity (302.0 mA h g−1 at 0.15 A g−1), excellent rate performance (159.9 mA h g−1 at 3 A g−1) and superior cycling stability. Furthermore, the results of ex situ characterization analysis reveal that H+ and Zn2+ insertion/extraction both occur in MnOx/PPy particles during the discharging/charging process, while only Zn2+ insertion/extraction occurs in the PPy electrode. This work develops an efficient one-step synthesis method for large scale production of manganese-based materials/conducting polymers as the cathode for ZIB application, and provides an insight into its energy storage mechanism.


 Please wait while we load your content...
Please wait while we load your content...