One-pot kinetic resolution–Mitsunobu reaction to access optically pure compounds, using silver salts in the substitution protocol†
Abstract
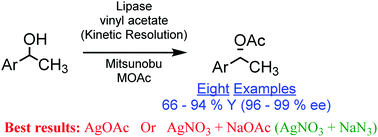
A practical method is developed to access chiral arylalkyl carbinols with a high yield from racemic alcohols. A one-pot enzyme mediated Kinetic Resolution followed by Mitsunobu esterification of the unreacted enantiomer of alcohol with metal acetate results in a nearly complete formation of chiral acetate. Substitution with AgOAc was found to be the most efficient, and the use of sub stoichiometric amounts of AgNO3 and excess of NaOAc affords comparable results; the protocol was further extended to introduce azide as a nucleophile.


 Please wait while we load your content...
Please wait while we load your content...