Synthesis of dibutyl-trimethylsilanylmethyl-amine and its application towards SO2 absorption with phase change behaviors†
Abstract
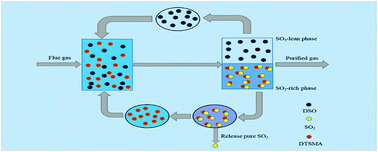
The enforcement of environmental law worldwide has attracted considerable attention in recent years towards capturing sulfur dioxide (SO2). The phase change absorption has turned out to be an effective way due to its industrially scalable characteristics. Herein, an aminosilane absorbent, namely dibutyl-trimethylsilanylmethyl-amine (DTSMA), was facilely synthesized by a substitution reaction of chloromethyltrimethylsilane and di-n-butylamine. The phase-change absorption performance of SO2 was systematically studied by using dimethyl silicone oil (DSO) as the solvent. The viscosity of pure DTSMA was only 1.29 mPa s, which is much lower than those of absorbents such as ionic liquids and deep eutectic solvents reported elsewhere, and the absorption product is found to be a charge transfer complex DTSMA SO2. The absorption capacity of DTSMA can reach 1.90 and 0.57 mol SO2 per mol DTSMA under 1 and 0.02 atm at 30 °C, respectively. In particular, the DTSMA/DSO solution can be not only almost regenerated within just 60 minutes at 100 °C, but also exhibited selective absorption towards SO2. All the results indicated that the synthesized DTSMA absorbent has enormous potential for application in capturing SO2 with benign circular desorption performance.


 Please wait while we load your content...
Please wait while we load your content...