Origin of stereoselectivity in an isothiourea catalyzed Michael addition reaction of aryl ester with vinyl disulfone†
Abstract
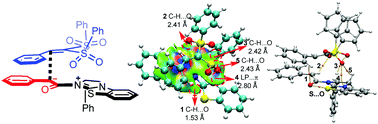
The general mechanism of the addition reaction of aryl esters with vinyl disulfone catalyzed by isothiourea was studied using density functional theory (DFT), and the origin of stereoselectivity and the role of the catalyst were discussed. The computed results indicate that the reaction can be divided into three stages, including the formation of an enolate ammonium intermediate, the Michael addition reaction, and dissociation of the catalyst. Among them, the formation of a C–C bond via Michael addition is identified as the stereoselectivity-determining step. The key factors that determine the stereoselectivity were further confirmed by non-covalent interaction (NCI) and atoms-in-molecules (AIM) analyses. In addition, the global reaction index (GRI) analysis shows that the main function of the catalyst is to improve the nucleophilicity (N) of the aryl ester to promote the addition reaction with vinyl disulfone. The gained insights will provide guiding information for the rational design of these kinds of reactions.


 Please wait while we load your content...
Please wait while we load your content...