Copper(ii) ions supported on functionalized graphene oxide: an organometallic nanocatalyst for oxidative amination of azoles via C–H/C–N bond activation†
Abstract
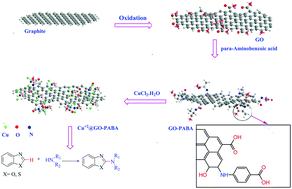
Graphene oxide (GO) was chemically modified with para-aminobenzoic acid (PABA) to immobilize copper(II) ions on its surface and used as a nanocatalyst for the oxidative C(sp2)–H bond amination reaction. A practical method to prepare Cu2+ supported on para-aminobenzoic acid grafted on GO was reported. The prepared Cu2+@GO/PABA was characterized by FT-IR, XRD, SEM, AFM, TEM, UV-Vis, and ICP techniques. The results showed that the morphology, distribution, and loading of copper ions could be well-adjusted by grafting of PABA on GO. Moreover, just 2 mol% of Cu2+@GO-PABA could catalyze the C–H activation reaction of benzoxazole and benzothiazole with secondary amines in >94% yields. Also, the catalyst showed very good recyclability and much less leaching of the Cu into the reaction solution. The high activity of Cu2+@GO-PABA can be ascribed to the good synergistic effects of Cu2+ and para-aminobenzoic acid grafted on graphene oxide.


 Please wait while we load your content...
Please wait while we load your content...