Theoretical study on the adsorption and catalytic degradation mechanism of sulfacetamide on anatase TiO2(001) and (101) surfaces†
Abstract
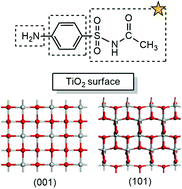
Density functional theory (DFT) calculations are used to explore the adsorption characteristics and degradation mechanism of sulfacetamide on anatase TiO2(101) and (001) surfaces. The most probable adsorption configuration was firstly determined by comparing the adsorption energies and structural bond lengths before and after adsorption. After adsorption, some of the bond lengths are elongated. We have proposed about six possible degradation pathways of sulfacetamide, such as branch chain breaking, benzene ring dihydroxylation, amino substitution and sulphur nitrogen bond breaking. And the relevant degradation processes are studied in detail. Based on the calculated results, we conclude that the TiO2(001) surface is more suitable for the degradation of sulfacetamide considering its much smaller energy barriers in several degradation paths than the TiO2(101). Among all the six possible degradation paths, we find that the hydroxyl radical attacks of C(1), C(3) and N(6) atoms of the (001) surface and the C(4) atom of (101) surface sulfacetamide are most probable reaction pathways due to their much smaller energy barriers, i.e. 7.7, 26.1 kcal mol−1 and 14.6, 23.4 kcal mol−1 indicating that these reactions can easily take place in room temperature. Our computational results are not only consistent with the experimental results of sulfacetamide degradation, but also reveal the possible microscopic mechanisms of the photocatalytic reaction.


 Please wait while we load your content...
Please wait while we load your content...