A rod-like hexanuclear nickel cluster based on a bi(pyrazole-alcohol) ligand: structure, electrospray ionization mass spectrometry, magnetism and photocurrent response†
Abstract
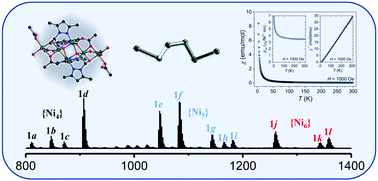
The reaction of one equivalent of 1,2-bis-(3,5-dimethylpyrazol-1-yl)-ethane-1,2-diol (H2bdped) with two equivalents of Ni(OAc)2·4H2O afforded a hexanuclear nickel(II) cluster [Ni6(bdped)2(OAc)8(H2O)4]·6EtOH (SD/Ni6a). The single-crystal X-ray diffraction (SCXRD) structural analysis reveals the one-dimensional zig-zag arrangement of six Ni(II) atoms that are in a slightly distorted octahedral coordination geometry. Two bdped2− ligands show folded configurations to coordinate to Ni(II) atoms. Interestingly, the multi intra- and inter-cluster hydrogen bonds in and between the clusters could be favorable for the stability of the cluster and the cluster packing in the crystal. Electrospray ionization mass spectrometry (ESI-MS) reveals that the Ni6 core structure keeps intact in solution and coexists with some fragments of Ni4 and Ni5 species. This fragmentation should be occurring in solution instead of the gas phase as indicated by in-source voltage dependent ESI-MS. Furthermore, SD/Ni6a displayed ferromagnetic exchange behavior arising from the interactions between the nickel(II) centers. UV-vis absorption presents typical Ni(II) species with octahedral coordination geometry and the photocurrent measurement gave fast and stable responses upon visible light illumination.


 Please wait while we load your content...
Please wait while we load your content...