Novel flavan-3-ol-glutathione conjugates from the degradation of proanthocyanidins as highly bioactive antioxidants†
Abstract
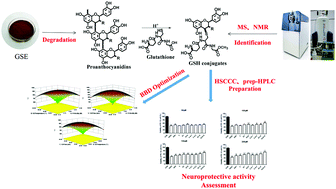
A new degradation method was developed for converting polymeric proanthocyanidins into highly bioactive flavan-3-ol derivatives in the presence of glutathione (GSH) as a nucleophile. Combining single-factor experiments and Box–Behnken factorial design (BBD), optimized degradation conditions were established. Grape seed proanthocyanidins were reacted with GSH at a ratio of 1 : 3 with 0.2 M methanolic HCl, a temperature of 55 °C and a reaction time of 30 min. The degradation products were largely isolated and prepared by one-step high-speed counter-current chromatography (HSCCC) and preparative HPLC. Three monomeric proanthocyanidins and four novel GSH conjugates were obtained with a high yield (85%) and high purity (over 91%). Also, the protective effects of all these compounds against H2O2-induced oxidative stress in PC-12 neuroblastoma cells were investigated. GSH conjugates were shown to be better at increasing the antioxidative effect than their underivatized monomers and GSH, indicating their better neuroprotective effects against oxidative stress.


 Please wait while we load your content...
Please wait while we load your content...