Supramolecular and theoretical perspectives of 2,2′:6′,2′′-terpyridine based Ni(ii) and Cu(ii) complexes: on the importance of C–H⋯Cl and π⋯π interactions†
Abstract
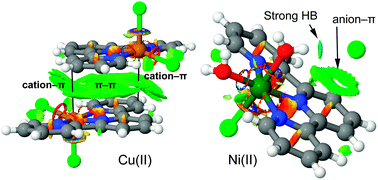
Two new complexes [Ni(L)Cl(H2O)2]Cl (complex 1) and [Cu(L)Cl2] (complex 2) [L = 2,2′:6′,2′′ terpyridine] have been synthesized and characterized by single crystal X-ray analysis. The noncovalent interactions and supramolecular assemblies observed in the crystal packing of both complexes have been described focusing on non-traditional C–H⋯Cl and ‘traditional’ O–H⋯Cl hydrogen bonding interactions along with π⋯π interactions. A DFT study has been carried out to analyze the π⋯π, hydrogen bonding interactions with their rationalization and characterization using molecular electrostatic potential (MEP) surfaces and NCI plot computational tools.


 Please wait while we load your content...
Please wait while we load your content...