An effective photothermal dual-responsive Pd1Cu4/CexOy catalyst for Suzuki–Miyaura coupling reactions under mild conditions†
Abstract
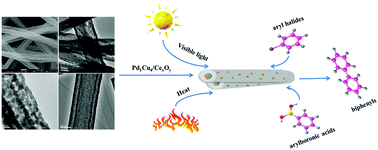
The Suzuki–Miyaura coupling reaction is one of the effective methods for forming C–C bonds in modern organic synthesis. However, most of the current catalysts can only effectively catalyze the aryl iodides and aryl bromides, and the catalytic activity for the inexpensive aryl chlorides is relatively low. Herein, the Pd1Cu4/CexOy catalysts can not only exhibit excellent performance in the conventional thermal reactions of aryl bromides and arylboronic acids, but also effectively activate aryl chlorides in Suzuki–Miyaura coupling reactions under visible light irradiation at room temperature. The CexOy carriers can provide photogenerated electrons to enrich the electron density of the Pd nanoparticles, which can effectively activate the strong C–Cl bonds of the aryl chlorides. Meanwhile, the photogenerated holes of CexOy can activate arylboronic acids. The presence of Pd and Cu2O can further enhance the absorption of visible light by CexOy. Catalysts prepared in this work could provide a great promise for using inexpensive metals to synergistically catalyze Suzuki–Miyaura coupling reactions under mild conditions.


 Please wait while we load your content...
Please wait while we load your content...