Oxidative desulfurization of dibenzothiophene catalyzed by molybdenum dioxide immobilized on zirconia-modified silica†
Abstract
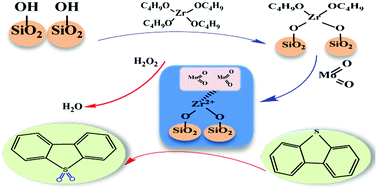
MoO2/ZrO2–SiO2 catalysts were successfully synthesized using a facile calcination route, and their performance in the oxidative desulfurization process was studied. Experimental results revealed that 5% MoO2/ZrO2–SiO2 exhibited the highest catalytic activity. The properties of the catalysts were investigated using methods such as X-ray photoelectron spectroscopy, Fourier-transform infrared and Transmission electron microscopies. X-ray diffraction confirmed that MoO2 was present in the ZrO2–SiO2 support. Temperature programmed desorption of NH3 proved that the MoO2/ZrO2–SiO2 catalyst has more acid sites. The reaction conditions were optimized in the oxidative desulfurization process. The sulfur removal rate was 99.96% under the optimum reaction conditions of 0.06 g catalyst, 298 K, and O/S = 4, for 30 min. After eight cycles, the activity of the catalyst decreased to 88.65%.


 Please wait while we load your content...
Please wait while we load your content...