Changing stereoselectivity and regioselectivity in copper(i)-catalyzed 5-exo cyclization by chelation and rigidity in aminoalkyl radicals: synthesis towards diverse bioactive N-heterocycles†
Abstract
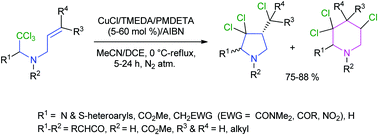
The work reveals that a chelate-type interaction in the transition state of a β-aminoalkyl radical in a copper(I)-catalyzed 5-exo-trig radical cyclization step changes the usual stereochemistry of the NH-pyrrolidine ring predicted by the Beckwith–Houk transition state model. In contrast, the rigidity in the fused β-aminoalkyl radical changes the Baldwin's predicted 5-exo to 6-endo cyclization mode, preferentially forming a piperidine ring over a pyrrolidine ring via a geometrically constrained transition state. The resultant diverse NH-pyrrolidines, pyrrolines and piperidines are sources of the bioactive natural product roseophilin and the drug Ritalin among others.


 Please wait while we load your content...
Please wait while we load your content...