Design and synthesis of a novel coumarin-based framework as a potential chemomarker of a neurotoxic insecticide, azamethiphos†
Abstract
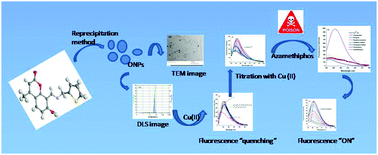
Selective affinity of a novel coumarin-functionalized fluorescent sensor, 8-((E)-((thiophen-2-yl)methylimino)methyl)-7-hydroxy-4-methyl-2H-chromen-2-one (L), to copper(II) ions via fluorescence quenching in HEPES buffer at pH 7.4 has been demonstrated. This coordination chemistry between the coumarin-based ligand L and copper(II) ions has been subsequently exploited for the generation of a unique chemical ensemble, L·Cu2+, which qualifies the former as a sensitive and selective fluorogenic sensor for the toxic organophosphate pesticide azamethiphos in aqueous medium. The sequestering of the native fluorescence of receptor probe L upon entrapment of copper(II) within the former was altered upon sequential administration of azamethiphos into the environment of the host complex, L·Cu2+. The physico-chemical interactions between the sensor complex L·Cu2+ and azamethiphos, as corroborated by 31P NMR studies and fluorescence spectroscopy, serve as the basis for ideal chemical discrimination of azamethiphos, amongst a pool of several ecotoxic organophosphate pesticides.


 Please wait while we load your content...
Please wait while we load your content...