Tracking ion intercalation into layered Ti3C2 MXene films across length scales†
Abstract
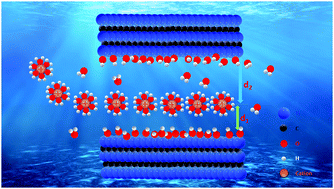
Enhancing the energy stored and power delivered by layered materials relies strongly on improved understanding of the intricate interplay of electrolyte ions, solvents, and electrode interactions as well as the role of confinement. Here we report a highly integrated study with multiscale theory/modelling and experiments to track the intercalation of aqueous Li+, Na+, K+, Cs+, and Mg2+ ions into Ti3C2 MXene. The integrated analysis of experiments assisted by theory/modelling allows for a deep understanding of energy storage processes highlighting the importance of the dynamics of cations, their positionings between MXene sheets, their effects on mechanical properties and capacitive energy storage. Computational simulations and operando calorimetry measurements prove the processes involving cation dehydration and H+ rehydration, showing a good correlation for heat variations between experiments and theory. Operando liquid AFM mapped energy dissipation of ions appears non-uniformly across the MXene surface, indicating heterogeneities of ions inside the MXene and confirming partially the ion behaviour obtained in theory. We directly demonstrate that the average distance between the cation and MXene surface follows a modified two-sided Helmholtz model when plotted versus the open circuit potential capacitance, revealing a different electrical double layer mechanism in confinement. This new fundamental understanding lays the foundation for improved functional devices utilizing electrodes and membranes made of two-dimensional materials.


 Please wait while we load your content...
Please wait while we load your content...