Reducing the charge overpotential of Li–O2 batteries through band-alignment cathode design†
Abstract
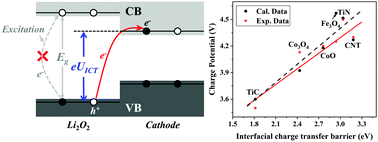
The rechargeable aprotic Li–O2 batteries, of much interest because of their high energy capacity, suffer from many challenges, one of which is the large overpotential resulting in low efficiency and some detrimental side reactions. Although porous cathodes with electrocatalytic activity that have reduced overpotential have been designed by improving the Li2O2 decomposition kinetics or regulating the discharge products, achieving an ideal charge potential of <3.5 V still faces significant challenges because the origin of high charge potential is poorly understood. Here, we revealed that the interfacial charge transfer at a hetero-structural Li2O2@cathode makes a major contribution to the overpotential through comprehensively studying Li+/O2 desorption and charge transfer kinetics in theory and experiments. A strategy of band alignment was proposed to reduce interfacial charge transfer and thus the overpotential. High-throughput calculations were performed to screen effective cathodes, of which the calculated charge potentials are in good agreement with the available experimental data. According to the screened cathode materials, 3d-metal carbides/nitrides with a low-valence-state metal and noble-metal oxides with a high-valence-state metal exhibit high electrochemical activities for reducing charge potential in Li–O2 batteries. Unreported cathodes such as MnN and Cr2O3 are predicted as high-activity cathodes with low charge potentials of <3.5 V.


 Please wait while we load your content...
Please wait while we load your content...