Zero valent iron complexes as base partners in frustrated Lewis pair chemistry†
Abstract
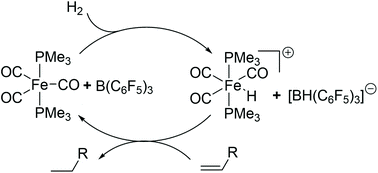
The prototypical iron(0) complex [Fe(CO)3(PMe3)2] (1) forms a frustrated Lewis pair (FLP) with B(C6F5)3 (BCF). In this FLP, the iron complex acts as the Lewis base partner, and the borane as the Lewis acid partner. This FLP is able to cleave H–H, H–Cl, H–O and H–S bonds in H2, HCl, H2O and HSPh. The FLP 1/BCF is shown to catalyze the hydrogenation of alkenes under mild conditions, where terminal alkenes are preferentially reduced. Mechanistic studies using D2 gas suggest that a branched intermediate in an alkene insertion cycle or an ionic cycle is favored for this catalytic reaction.


 Please wait while we load your content...
Please wait while we load your content...