Comparative bindings of lactones, lactide, and cyclic carbonates: experimental insights into the coordination step of polymerization†
Abstract
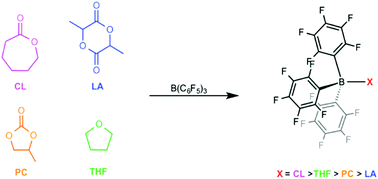
Comparative bindings of several renowned monomers were investigated experimentally using B(C6F5)3 as a Lewis acid model for the coordination step in ring-opening polymerization. A complete series of the X-ray crystal structures of the B(C6F5)3 adducts with the monomers was reported. The X-ray structural studies and spectroscopic data revealed a coordination strength in the order lactones > tetrahydrofuran > cyclic carbonates > lactide.


 Please wait while we load your content...
Please wait while we load your content...