Isomers in chlorido and alkoxido-substituted oxidorhenium(v) complexes: effects on catalytic epoxidation activity†
Abstract
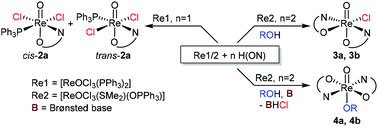
The syntheses and characterizations of oxidorhenium(V) complexes trans-dichlorido [ReOCl2(PPh3)(L1a)] (trans-2a), cis-dichlorido [ReOCl2(PPh3)(L1b)] (cis-2b) and ethoxido-complex [ReO(OEt)(L1b)2] (4b), ligated with the dimethyloxazoline-phenol ligands HL1a and HL1b are described. The bidentate ligand HL1a (2-(4,4-dimethyl-4,5-dihydro-1,3-oxazol-2-yl)-phenol) is unsubstituted on the phenol ring; ligand HL1b (2-(4,4-dimethyl-4,5-dihydro-1,3-oxazol-2-yl)-4-nitrophenol) contains a nitro group in para-position to the hydroxy group. In the reaction of precursor complex [ReOCl3(PPh3)2] and HL1a the two stereoisomers cis/trans-2a, with respect to chlorido ligands, are formed. The solid state structures of both isomers cis- and trans-2a were determined by single crystal X-ray diffraction analysis. In contrast, with ligand HL1b, only the cis-isomer cis-2b was obtained. Ethoxido-complex 4b is exclusively obtained when precursor [ReOCl3(OPPh3)(SMe2)] is reacted with 2 equiv. of HL1b in ethanol in the presence of the base 2,6-dimethylpyridine (lutidine). If no lutidine is added, chlorido-complex [ReOCl(L1b)2] (3b) is obtained. Complexes [ReOCl2(PPh3)(L1a)] (cis/trans-2a), [ReOCl2(PPh3)(L1b)] (cis-2b), [ReO(OMe)(L1a)2] (4a) and [ReO(OEt)(L1b)2] (4b) were tested as homogeneous catalysts in the benchmark reaction of cyclooctene epoxidation. The influence of isomerism and effects of ligand substitutions on catalytic activity was investigated. Based on the time-conversion plots it can be concluded that cis/trans-isomerism does not influence catalytic activity, but electron-withdrawing substituents, as in cis-2b, 3b and 4b, show a beneficial effect.


 Please wait while we load your content...
Please wait while we load your content...