Palladium aminopyridine complexes catalyzed selective benzylic C–H oxidations with peracetic acid†
Abstract
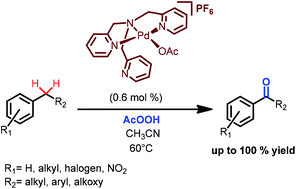
Four palladium(II) complexes with tripodal ligands of the tpa family (tpa = tris(2-pyridylmethyl)amine) have been synthesized and X-ray characterized. These complexes efficiently catalyze benzylic C–H oxidation of various substrates with peracetic acid, affording the corresponding ketones in high yields (up to 100%), at <1 mol% catalyst loadings. Complex [(tpa)Pd(OAc)](PF6) with the least sterically demanding ligand tpa demonstrates the highest substrate conversions and ketone selectivities. Preliminary mechanistic data provide evidence in favor of metal complex-mediated rate-limiting benzylic C–H bond cleavage by an electron-deficient oxidant.


 Please wait while we load your content...
Please wait while we load your content...