Mitochondria-localizing dicarbohydrazide Ln complexes and their mechanism of in vitro anticancer activity†
Abstract
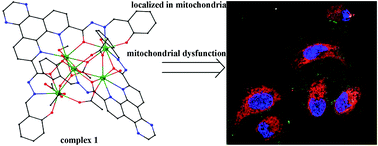
In this study, two novel organic ligands, bis(salicylaldehyde)pyrazino-1,10-phenanthroline-7,10-dicarbohydrazide (L) and bis(salicylaldehyde)-1,10-phenanthroline-7,10-dicarbohydrazide (L1), were synthesized. These ligands were used to react with lanthanide(III) acetate to obtain complexes 1–6, namely, [Dy5(L)2(CH3COO)5(CH3OH)(μ3-OH)(μ2-OH)(H2O)]·2CH3OH (1), [Tb5(L)2(CH3COO)5(CH3OH)(μ3-OH)(μ2-OH)(H2O)]·3CH3OH (2), [Gd5(L)2(CH3COO)5(CH3OH)(μ3-OH)(μ2-OH)(H2O)]·3CH3OH (3), [Dy5(L1)2(μ2-OH)(μ3-OH)(CH3COO)5(CH3OH)(H2O)2]·2H2O (4), [Dy5(L1)2(μ3-OH)(CH3COO)6(CH3OH)3]·CH3OH (5), and [Dy5(L1)2(μ2-OH)2(μ3-OH)(CH3COO)4(CH3OH)(H2O)2]·CH3OH (6). Fluorescence studies demonstrated that complexes 1–6 show appreciable fluorescence in the yellow-green region. In vitro antitumor screening revealed that complex 1 exhibits better inhibitory activities than the commercial anticancer drug cisplatin against SK-OV-3 and A549 tumor cell lines, with IC50 values of 8.09 ± 1.25 and 13.26 ± 0.39 μM, respectively. All six complexes showed low cytotoxicity toward normal human liver HL-7702 cells compared with cisplatin. Complexes 1 and 3 induced the highest apoptosis rate of SK-OV-3/DDP cells. They also bind to DNA via an intercalative mode with the binding constant Kq values of 1.6 × 104 and 1.19 × 104 L mol−1, respectively. Confocal fluorescence imaging ascertained that complexes 1 and 3 are primarily localized in the mitochondria. Further studies revealed that these complexes trigger SK-OV-3/DDP cell apoptosis via a mitochondrial dysfunction pathway, which is probably caused by the reduction of the mitochondrial membrane potential and the induction of reactive oxygen species production.


 Please wait while we load your content...
Please wait while we load your content...