Hierarchical porous ZIF-8 for hydrogen production via the hydrolysis of sodium borohydride†
Abstract
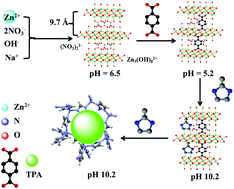
Hydrides show good performance for hydrogen gas storage/release. However, hydrogen gas release from hydrides via hydrolysis is a slow process and thus requires a catalyst. Herein, terephthalic acid (TPA) is used for the synthesis of a hierarchical porous zeolitic imidazolate framework (HPZIF-8). A mechanistic study of materials synthesis involved an in situ synthesis of zinc hydroxide nitrate nanosheets with an interplanar distance of 0.97 nm. Terephthalic acid modulates the pH value of the synthesis solution leading to the formation of HPZIF-8 with the Brunauer–Emmett–Teller (BET) surface area, Langmuir surface area, and total pore size of 1442 m2 g−1, 1900 m2 g−1, and 0.69 cm3 g−1, respectively. The formed phases during the synthesis undergo fast conversion to HPZIFs at room temperature. The application of the prepared materials in the hydrolysis of NaBH4 is reported. Acidity plays an important role in the catalytic performance of the materials. ZIF-8 prepared using terephthalic acid shows high catalytic activity with a hydrogen rate of 2333 mLH2 min−1 gcat−1 (8046 mLH2 min−1 gZn−1). The material exhibits high catalytic activity without any deterioration of its performance for several uses during continuous NaBH4 feeding. There are no changes in the material's structure after catalysis indicating the high recyclability of the materials.


 Please wait while we load your content...
Please wait while we load your content...