Efficient and stable degradation of chlorobenzene over a porous iron–manganese oxide supported ruthenium catalyst†
Abstract
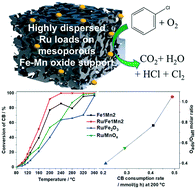
The development of efficient and stable catalysts for the degradation of chlorinated volatile organic compounds (CVOCs) is a hot topic of global concern. However, both reactivity and the lifetime of catalysts are severely hampered by the Cl species. This work focuses on the low-temperature catalytic combustion of chlorobenzene (CB) under industry-relevant reaction conditions using a Ru–Fe–Mn catalysis system with high activity and stability. Ru supported on mesoporous Fe–Mn oxide was prepared and characterized by various techniques, such as XRD, N2 sorption, TEM, H2-TPR, NH3-TPD and EPR measurements, which identified favourable features, including small particle size and the ameliorative metal–support effect, that enhance the catalytic activity and material stability. The interaction between Ru, Fe and Mn species on the surface resulted in the formation of Ru–O–Mn and Ru–O–Fe structures. The Ru–O–Mn linkage promoted reducibility and surface-adsorbed oxygen, and Ru–O–Fe facilitated oxygen vacancies and acidity. In CB streams, the Ru/Fe1Mn2 sample performed the best with T50 and T90 of 158 and 197 °C, respectively, under 600 ppm CB at a GHSV of 20 000 h−1. During the stability tests, the catalyst was stable even in humid conditions, showed high selectivity and hardly formed polychlorinated congeners, as proven by GC/MS measurements. The reaction pathway was investigated by in situ DRIFTS, which revealed that the surface hydroxyl groups and Lewis acid sites synergistically reacted with CB to form HCl and benzene. The aromatic ring reacted with active oxygen to generate intermediates, such as carboxyl, anhydride, formate and carbonates, which were further oxidized by the surface oxygen species into CO2 and H2O.


 Please wait while we load your content...
Please wait while we load your content...