Insights into C–O insertion in a carbene/alkyne metathesis cascade reaction catalyzed by Rh2(OAc)4: a DFT study†
Abstract
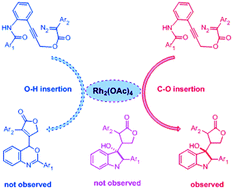
The reaction mechanisms of C–O insertion in the carbene/alkyne metathesis (CAM) cascade reaction of alkyne-tethered diazo compounds catalyzed by Rh2(OAc)4 are thoroughly studied with density functional theory (DFT) calculations. This is a detailed investigation into the CAM process, theoretical studies of which have rarely been reported before. The mechanism involves three key steps: CAM, cycloaddition and the H-transfer step, and the a priori order is the CAM step first, cycloaddition later and the H-transfer last. Meanwhile, the rate-determining step appears in the H-transfer step with a free energy barrier of 22.7 kcal mol−1. Interestingly, our study suggests the final product can act as a catalyst to make the product form easily. The origins of the stereoselectivity and chemoselectivity of the catalytic reaction are investigated by distortion/interaction analysis, non-covalent interaction (NCI) analysis, and NBO calculations. It is the steric hindrance and weak interaction of the phenyl groups that control the stereoselectivity to generate the R-configuration product. In addition, the higher energy barrier of the 1,4-H transfer step makes the chemoselectivity more distinct.


 Please wait while we load your content...
Please wait while we load your content...