Rotational spectra of van der Waals complexes: pyrrole–Ne and pyrrole–Ne2†
Abstract
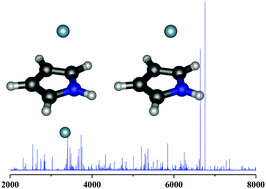
The van der Waals 1 : 1 and 1 : 2 adducts between the aromatic molecule pyrrole (Pyr) and the rare gas atom neon (Ne) have been investigated using a combination of chirped pulse Fourier transform microwave spectroscopy and quantum-chemical calculations. Rotational spectra of two and three isotopologues of Pyr–Ne and Pyr–Ne2, respectively, arising from the combinations of the 20Ne and 22Ne isotopes, were identified and a partial rs structure determined. Unusual spectral intensities have been observed with a significant enrichment of heavier isotopic species in the jet molecular expansion. The observed rotational constants of Pyr–Ne are consistent with a nearly symmetric prolate top with the Ne atom located above the plane of pyrrole. The trimer presents C2v symmetry with the Ne atoms located one on each side of the ring plane. The experimental 14N nuclear quadrupole coupling constants have been determined for all the isotopologues of Pyr–Ne and Pyr–Ne2 complexes. Similar values to those of isolated pyrrole have been found, which suggests that the electrical gradient field of pyrrole does not change much upon complexation. The observed spectroscopic parameters have been compared with those of other aromatic-rare gas complexes.


 Please wait while we load your content...
Please wait while we load your content...